The sun is a massive hydrogen bomb in the sky. It puts out energy, in the form of electromagnetic radiation, a small sliver of which comes our way. The blindingly bright portion that’s in the visible range enables us to see things. On either side of the visible frequencies, there’s ultraviolet (that which gives you a suntan and skin cancer), which has a higher frequency and energy level than visible light, and infrared, which is comparatively pusillanimous. The earth radiates some of the heat back towards the cold blackness of space, but most of what it radiates back is infra-red, which is low-energy enough that any gas whose molecules have more than two atoms–so-called greenhouse gases–tend to absorb it, and so get a little warmer. As a result, most of the earth’s heat radiation warms our own the atmosphere instead of escaping back into space. CO2 is one of the greenhouse gases (along with water vapor, methane and ozone).
The amount of CO2 in the atmosphere has increased from about 280 parts per million before the industrial revolution to slightly more than 400 ppm now, so it’s a growing problem. This is not to say that it hasn’t been higher. About 50 million years ago it was at about 3,500 ppm, and there were palm trees, they say, at the earth’s poles. Then a million years later, with the help of a freshwater fern called Azolla, which dragged huge amounts of carbon to the bottom of the Arctic, levels plummeted to about 650ppm, and ice appeared. That climate and CO2 levels are related it seems strange to deny.
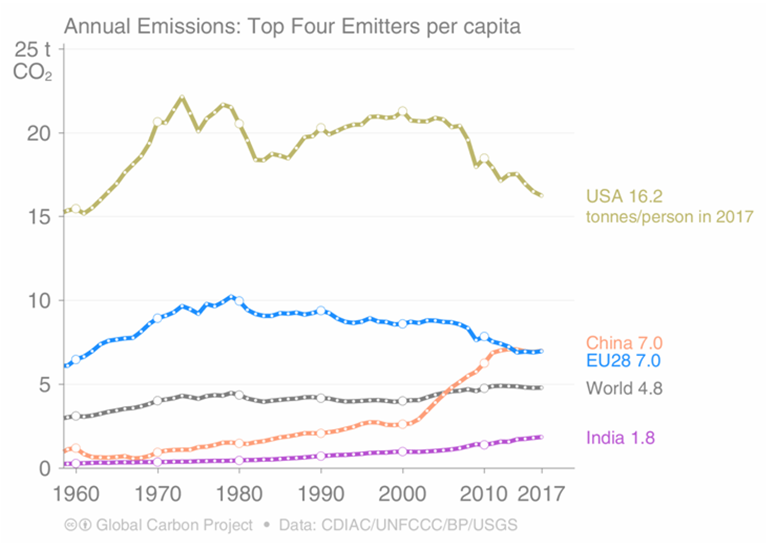
With our cars, planes, electrical gizmos and general industrial busy-ness, we are pumping about 37 billion tons of CO2 into the atmosphere every year. That’s more than 60% higher than it was 30 years ago. On a per-person basis, the USA stands out as the indisputable champion emitter.
Despite its bad rep, though, CO2 is an essential stage in earth’s eco-cycle, whereby plant fuel is eaten (or burned), returned to the atmosphere as CO2 and then turned back into fuel again by plants, with the help of sunlight and water. It’s a kind of mediator on which the whole multitude of carbon-based life-forms relies. As well as plants taking CO2 back out of the air, the world’s oceans and lakes do their part in trying to restore the balance, absorbing huge amounts. The effect of this is increased acidity, which causes some mineral-dependent life-forms, like some corals, crustaceans and shellfish, to become a little thin-skinned—enough, often, to kill them off.
Unfortunately, there aren’t enough plants in the world, and the oceans are not big enough, to keep the stuff from accumulating in the atmosphere. Those people who think that’s not a good thing are trying to figure out ways to “sequestrate” some of the CO2 and so ameliorate the problem. So far, researchers haven’t come up with much, and some of the proposed solutions, like shoving it deep down underground—for instance, back into the spaces where oil and gas used to be—seem unrealistically wistful, if not downright scary.
So, as a gas, CO2 is a rather depressing subject. But CO2 isn’t always a gas. If you take the temperature down to about -80°C (about -110°F), it turns from gas to solid—a snow-like material called dry ice—without any liquid phase in between. At rather high pressure, CO2 becomes a glass, something it has in common with silicon dioxide (which is the main component of everyday glass) and germanium dioxide, the oxides of two elements in the same periodic group as carbon (Group 14 by conventional chemists, but Group IV in the lingo of semiconductor physicists). By high pressure, though, we’re talking about 400,000 times atmospheric pressure, which would be roughly six million pounds per square inch. To achieve that kind of pressure you need what they call a diamond anvil. And some courage, probably. So CO2 will not be replacing our window panes any time soon.
Maybe the most interesting form it can take, though, is as a supercritical liquid. It sounds like something out of a Marvel comic, and it practically is.
When you raise the temperature of carbon dioxide above 88°F and pressurize it to above 73 atmospheres, it shape-shifts into a sort of mystical, other-worldly state where it is neither a solid, nor a gas, nor a liquid (nor glass). Supercritical CO2 is heavy like a liquid but as penetrating as a gas, with surprisingly weird and useful properties. In this artificially created condition, CO2 can be very useful stuff.
Mainly, it’s a safe alternative to a lot of conventional solvents, particularly organic solvents, many of which, apart from being dangerously flammable, are carcinogenic, cause birth defects or are toxic to the central nervous system. A lot of them also, if they haven’t polluted the land or water, or caused a giant explosion, are volatile compounds which evaporate into the atmosphere and cause all kinds of trouble, including poking holes in the ozone layer or trapping heat as greenhouse gases. Millions of people are exposed to these organic solvents at work, where they’re used in countless industrial processes.
True, sCO2 turns into a greenhouse gas when it returns to its usual gaseous state, but if it was taken from the atmosphere in the first place there’s no net effect when you put it back. Because it’s contained in high-pressure vessels, it can’t very well poison anyone (and wouldn’t anyway, except in suffocatingly high doses like what might happen if you “sequestered” it underground) and there’s no way you can set it alight.

One of the first things supercritical CO2 was used for was in extracting the caffeine from coffee, for example. Among other solvents that have been used for this are methylene chloride, ethyl acetate, methyl acetate, ethylmethylketone and trichloroethane, the mere sounds of which are enough to start tumors sprouting in your liver. sCO2 has also been used for dry cleaning, in preference to the toxic and volatile “perc” (perchloroethylene), which is the usual stuff they use to get dirt and stains out of those clothes you are too fastidious to put in your washing machine.
Another big advantage is that once you take the pressure off, the CO2 just disappears into the air. With perc, for example, it is a long and tedious process to flush out and recycle the solvent to prevent it contaminating the atmosphere. (Recycling and containing perc is a relatively modern innovation: originally the boffins reckoned it was harmless; it is now classified as “moderate to low” toxicity, but Perc machines will be banned in California from 2023).
The solvent power of supercritical CO2 can be manipulated quite easily: It is what the people who make up catchy neologisms call a “tunable” solvent. How well it dissolves stuff is partly determined by its density: the more dense it is, the better it is at dissolving things. The density of sCO2 is very sensitive to changes in temperature and pressure. So, unlike with conventional solvents, you can vary the solvent power a lot with relatively small tweaks in temperature and pressure. That means, for instance, that you can fine-tune it to extract one component out of a complex mixture and not another, which makes it very handy for industrial chemists. And it can exist at a relatively low temperature—lower than say, a typical July day in New York—which makes it less likely to impair the character of whatever it is that is being dissolved. For instance, sCO2 has been used by perfumiers to extract the fragrant compounds from fragile flower petals, to dissolve nicotine out of tobacco and hops in beer brewing, and to isolate the various oils in Cannabis sativa. It is a bit of a hassle, because you need (expensive) specialized equipment to pressurize the CO2, but there is that benefit that you don’t need to do a lot of cleanup afterwards, or worry that the extract will be contaminated by some dangerous carcinogen. Many of the results you’ll get on DuckDuckGo for ‘CO2 extraction’ (we don’t use Google if we can help it) are for machines to help you get the good stuff out of cannabis.
A most interesting emerging use for sCO2 is in power generation. Most power generation is done using steam turbines: steam from boiling water is used to turn a fan, which creates electricity by moving coils of wire through a magnetic field. Typical systems include multiple turbine stages (high, medium and low pressure), each with its own casing and pipe systems. sCO2, being much more dense than steam, only needs one turbine, and the saving in space and complexity is substantial: GE has built a prototype which is small enough to fit on a desktop—that’s about a tenth of the size of a comparable steam turbine—and generates enough electricity to power 10,000 homes.
 Public Domain
Public DomainAlso, whereas a conventional turbine takes a while to get up steam, sCO2 takes up heat much more quickly and can be powering the turbine in a couple of minutes, which means it might also be used instead of batteries for power storage. The efficiency of GE’s prototype was 50% (conversion of heat energy to electricity), which is above the rate for state-of-the-art steam turbines and well above the 33% global average for coal-fired power plants. If you could lift the average to 40%, you would cut CO2 emissions by a couple of gigatons each year, roughly equivalent to the amount belched out annually by India.
It would be poetic justice to use CO2 to cut CO2 emissions.






